A new paper in Science, co-authored by evolutionary biologist Debashish Bhattacharya, Department of Ecology, Evolution, and Natural Resources, helps to answer the question.

Debashish Bhattacharya
A major turning point in the history of life on Earth occurred about two billion years ago – the evolution of complex cells, the so-called eukaryotes. This was the foundational lineage that contained the first nucleus, an elaborate internal membrane system, and energy producing organelles referred to as mitochondria that powered the rise of algae, fungi, plants, and ultimately humans. But how did this quantum leap in evolutionary complexity happen?
This question has long been a source of controversy among “deep time” thinkers but has finally been clarified to a great extent, and the story goes as follows. Before eukaryotes, our planet belonged to morphologically simple prokaryotic forms that lacked the nucleus and the mitochondrion. Prokaryotes are divided into two domains of life, bacteria and archaea, which are distinguished by their genetic make-up and the type of membranes that surround their cells. Most scientists now believe that eukaryotes evolved from prokaryotic ancestors in a unique event. The consensus view is that the mitochondrion traces its origin to an alphaproteobacterium that was captured by an archaeal cell. Major questions that remain are how the ancestor of the mitochondrion entered the archaeal cell and how it managed to survive in the well-protected host cell cytoplasm.
A recent finding by a Swedish team of researchers provided exciting new clues to answer these questions. They discovered a novel class of archaea living at hydrothermal vents more than 3000 meters below sea level at the Arctic Mid-Ocean Ridge. These so-called “Lokiarchaeota” unexpectedly contain genes that are required for the process of endocytosis; i.e., the uptake of macromolecules and perhaps even entire cells from the environment. This discovery provided a straightforward solution to a long-standing riddle about how the mitochondrial ancestor gained access to the proto-eukaryotic cell. As now suggested by a team of U.S., French, and German researchers in an article published February 11 in Science (Ball et al. 2016), the mitochondrial ancestor might have simply entered its future host via a process that is related to phagocytosis in current-day eukaryotes (i.e., one cell ingests another leading to primary endosymbiosis).
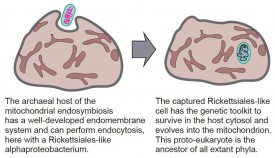 But how did it manage to survive inside the host? Additional clues come from the finding of alphaproteobacteria with large genomes that can live outside of cells but also act as energy-parasites within cells, similar to Rickettsia the agent of typhus. Intracellular bacterial pathogens such as the ancestors of Rickettsia and Chlamydia, which still parasitize humans today, were newcomers in the evolution of life on our planet because they relied entirely on the presence of an active phagocytosis machinery to penetrate the target cell. These intracellular bacterial pathogens evolved tools that prevent destruction by the array of defenses the host mounts when invaded by foreign bacteria. Therefore, the origin of eukaryotes likely involved a proto-eukaryote host derived from the Lokiarchaeota lineage that phagocytosed a large-genomed alphaproteobacterium adept at evading host defenses. Over time, the invader was harnessed by the host and became the mitochondrion.
But how did it manage to survive inside the host? Additional clues come from the finding of alphaproteobacteria with large genomes that can live outside of cells but also act as energy-parasites within cells, similar to Rickettsia the agent of typhus. Intracellular bacterial pathogens such as the ancestors of Rickettsia and Chlamydia, which still parasitize humans today, were newcomers in the evolution of life on our planet because they relied entirely on the presence of an active phagocytosis machinery to penetrate the target cell. These intracellular bacterial pathogens evolved tools that prevent destruction by the array of defenses the host mounts when invaded by foreign bacteria. Therefore, the origin of eukaryotes likely involved a proto-eukaryote host derived from the Lokiarchaeota lineage that phagocytosed a large-genomed alphaproteobacterium adept at evading host defenses. Over time, the invader was harnessed by the host and became the mitochondrion.
A similar but more complex scenario is suggested by Ball et al. (2016) for the endosymbiotic origin of the plastid in algae and plants that occurred about a billion years later. In this case, a tripartite symbiosis involving an intracellular pathogenic bacterium belonging to the Chlamydiales and a photosynthetic cyanobacterium jointly invaded the well-established eukaryotic host cell. The pathogen was eventually lost after donating key genes to the host, such as for photosynthetic carbon export and starch biosynthesis, and the cyanobacterium became the remarkable organelle that allows eukaryotes to convert sunlight into food and energy.
To summarize, the key advances made here are that we now have evidence for a common mechanism that explains the origin of the canonical eukaryotic organelles, the mitochondrion and the plastid. This process, primary endosymbiosis was made famous in modern times by Lynn Margulis. Beyond this step forward, we now suspect that intracellular bacterial pathogens were fundamental to both instances of organellogenesis. In the case of the mitochondrion the donor cell was itself a pathogen with a large genetic capacity that allowed it to evade host defenses, and for the latter the chlamydial pathogen sheltered the cyanobacterium that ultimately became the plastid.

